The medical device industry is today's high-tech industry and has always been valued by various countries. China's medical device industry started late, but it has developed rapidly. With the drive of the national economy and the further improvement of human medical needs, China’s medical devices must find new development patterns in the new situation and strive to steadily move forward.
Reviewing the prospects for the development of medical devices under the policy pattern
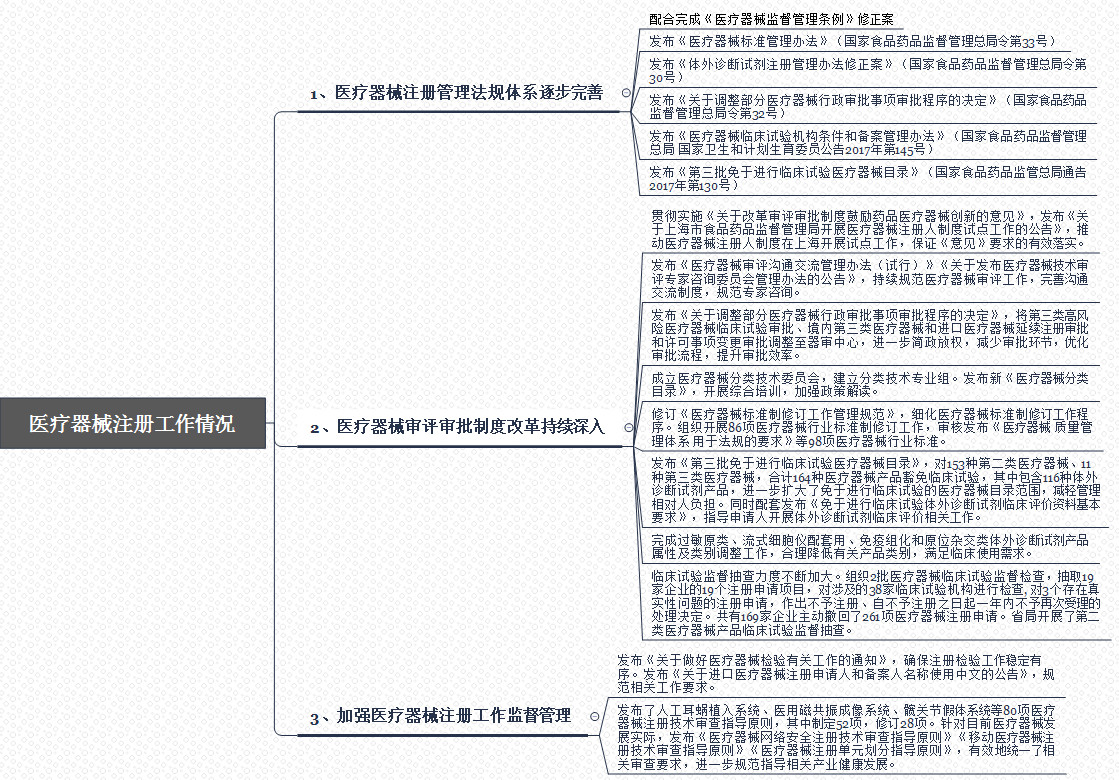
In recent years, with regard to the development of medical devices, the relevant agencies in the country have exerted their efforts with precision, constantly optimized and improved their policies, and have never been taken lightly in their supervision. The history of CFDA supervision is shown in the following diagram. Throughout 1998 to 2018, In the process, food and drug supervision is changing step by step, but the emphasis on medical devices has never been lowered.
CFDA regulatory history

Today (March 28), the General Administration of Administration issued the “2017 Medical Device Registration Work Report†(hereinafter referred to as the “Reportâ€), which summarizes the registration management and supervision of the medical device industry in 2017. We can see that China's medical device registration management. The regulatory system, review and approval system, and supervision and management system are gradually improving, providing basic guarantees for the development of medical devices. With the support of regulations and policies, the prospects for the development of medical devices can be expected.
Enlightenment of the Registration Pattern in 2017 to 2018
(I) Registration and Acceptance in 2017
In 2017, a total of 6,834 applications for registration of medical device registrations, continuation of registrations, and changes in licenses were accepted. Compared with 2016, registered applications decreased by 23.4%. A total of 8923 items were registered for the registration of medical device registrations, continuation of registrations, and licensing changes. Compared with 2016, the total number of registration approvals increased by 3.1%. From the data, it can be seen that there are relatively many third-type medical devices in the country. In addition, the provincial-level food and drug regulatory authorities approved a total of 18,582 domestic second-class medical device registrations. It can be seen that the domestic medical devices have a good development potential.

Approval of Medical Device Registration in 2017

The second type of medical device registration in each province
Looking from a longitudinal perspective, from 2013 to 2017, the approval of medical devices by the Directorate General showed a slight change. There was a slight increase in 2017 compared to 2016.

2013-2017 Approval of Registration

The type of registration is mainly the continuation of registration, and the applications for approval and acceptance are continuation applications, and the number of applications for first registration is relatively small.
2017 General Agency Medical Device Registration Application Type
The registered varieties were mainly medical devices, 4748 items were accepted, 5475 items were approved, 2086 items of in vitro diagnostic reagents were accepted, and 3178 items were approved.

Classification of Medical Device Registrations in 2017
(B) Domestic and imported major registered species
From the specific varieties, it can be seen that the registered quantity of the third type of medical devices in China is mainly: medical high-molecular materials and products, implant materials and artificial organs, injection and puncture devices, medical optical instruments, instruments and endoscope equipment, operating rooms, Emergency rooms, clinic equipment and appliances. The registered quantity of imported medical devices is mainly: implanted materials and artificial organs, medical optical instruments, instruments and endoscopic equipment, dental materials, medical polymer materials and products, and medical electronic equipment.
Can see the domestic market for medical polymer materials and products, implant materials and artificial organs, medical optical instruments, instruments and endoscopic equipment demand. Compared with 2016, the domestic medical high-molecular materials and products increased by 56.5%, jumping from the second place to the first place. The implanted materials and artificial organs dropped by 10.5%, ranking second in medical optics. Appliances, instruments and endoscope equipment are flat. Imported implants and artificial organs remained at the top position, down 5.1% year-on-year, medical polymer materials and products declined, and stomatologic material products increased significantly, replacing the operating room and emergency room. The top five equipment and equipment in the treatment room. It can be seen that in medical high-molecular materials and products, the domestic market is rapidly developing and has occupied a major position.

Domestic Tertiary Class Registration of Medical Devices Registers

Imported Medical Device Registered Bitmap
(III) 2017 Registration Number of Regions
From the distribution of major regions, we can see that our source of imports is mainly the United States. The third type of medical equipment in China is mainly developed in Beijing, Jiangsu, and Guangdong.

Imported medical device country registration bitmap

Registered provinces of the third category of medical device registration in China
(IV) Review, Approval and Approval of Innovative Medical Devices and other Products
In 2017, the General Food and Drug Administration approved the launch of some innovative medical device products. In the year, a total of 273 applications for special approvals for innovative medical devices were received, 323 reviews were completed (including applications for 2016), and 63 products were approved for special approvals for innovative medical devices. Twenty-two innovative products such as registered branch-type aortic stent grafts and delivery systems were approved. Among them, there were 4 active medical devices and 8 passive medical devices, which increased by 2 compared to 2016.
The core technologies of these innovative products have been patented by China's Patent Administration Department of the State Council. The main working principle/mechanism of the product has been pioneered in China and has significant clinical application value.

According to the overview of the approval format for medical device registration review in 2017, we can see that the pattern of domestic registration review in 2017 has a good trend. In many high-tech medical device products, the number of domestic registrations has surpassed imports, and the state supports policies for innovative products. Making more products appear or will appear on the market, it is worth looking forward to. In 2018, in the face of new domestic and international changes, the medical device industry should pay more attention to its own development and strive to allow China's high-value medical device industry to move from the stage of absorbing technological advantages of developed countries to its own stage of innovation.
Touch Panel For Iphone,Touch Screen Digitizer Panel,Touch Screen Panel For Iphone,Phone Touch Screen
Shenzhen Xiangying touch photoelectric co., ltd. , https://www.starstp.com